
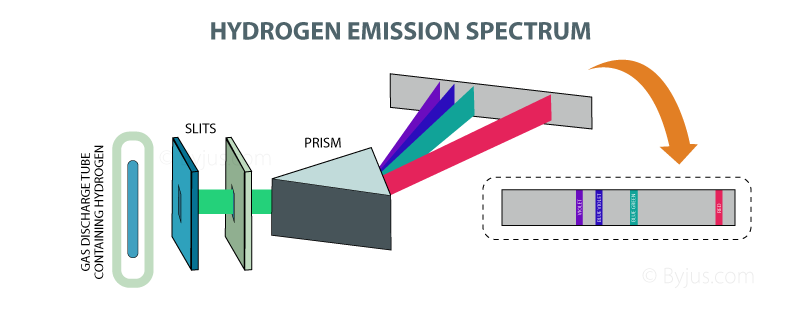
As a result each produces photons with different energy and so the line spectra for different elements will be different. The energy is lost in the form of photons of certain. This is not a continuous spectrum as only light of specific frequencies and specific colours are produced.ĭifferent types of atoms have different energy levels. Line Spectra are the wavelengths of light emitted from an element when its electrons lose energy. This causes line emission spectra to be produced, as shown below. Let’s understand why and how it radiates energy. This means that each electron transition will produce a photon of a different frequency and hence a different colour. During this process, the hydrogen atom’s electron emits EM radiation called the spectrum, which is a line spectrum. Interpret the hydrogen spectrum in terms of the energy states of electrons. Describe Rydberg's theory for the hydrogen spectra. There are multiple emission lines for hydrogen (and other elements) because an Bohr-model atom consists of orbits of many energy levels, which in turn allows. \(f\) is the frequency of light producedĪs the energy levels have different values, each of the possible electron transitions within an atom will produce a photon with a different energy. Overview To introduce the concept of absorption and emission line spectra and describe the Balmer equation to describe the visible lines of atomic hydrogen.If an electron moves from level \(E_\) the energy of the photon can be worked out using the following: The energy of the photon can be worked out using the equation Bohrs model of the atom accounted mathematically for the energy of each of the transitions shown. The amount of energy it loses will be equal to the difference in the energy levels it moves between.
HYDROGEN LINE EMISSION SPECTRUM SERIES
If an electron is in an excited state it can return to a lower energy level. In physics and chemistry, the Lyman series is a hydrogen spectral series of transitions and resulting ultraviolet emission lines of the hydrogen atom as an electron goes from n 2 to n 1 (where n is the principal quantum number ), the lowest energy level of the electron.


 0 kommentar(er)
0 kommentar(er)
